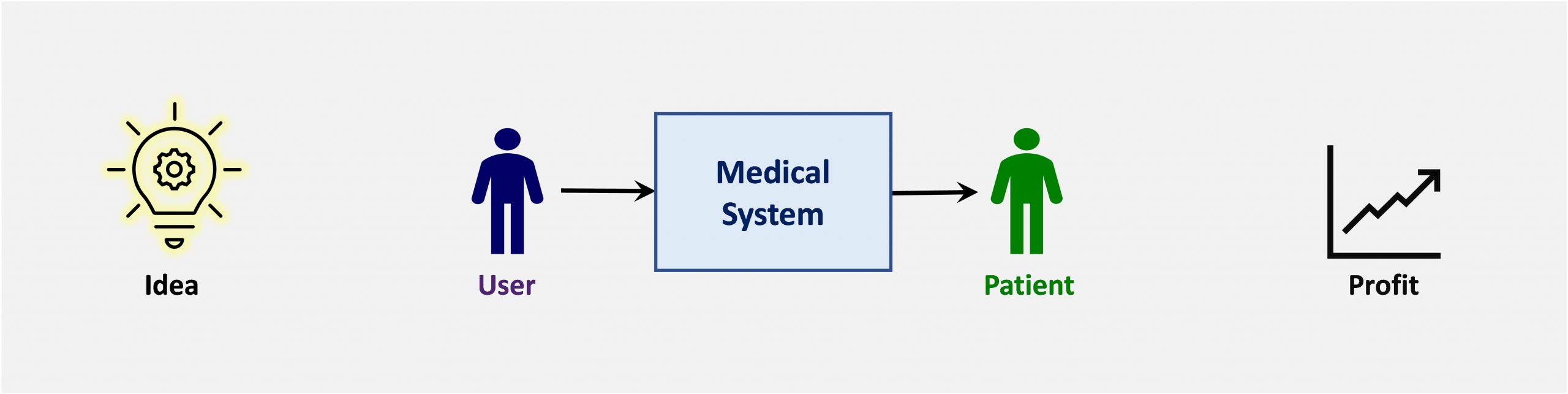
AganaMed LLC is a medical device consulting company that provides training and product development services for Medical Device Development and Due Diligence.
Expertise
Primary areas of expertise are:
- Design Controls – ISO 13485, FDA 21 CFR Part 820
- Risk Management – ISO 14971
- Quality Assurance – ISO 13485, FDA 21 CFR Part 820
- New Product Development – performance, profit, speed to market
- Business Process Optimization – effectiveness, efficiency, compliance
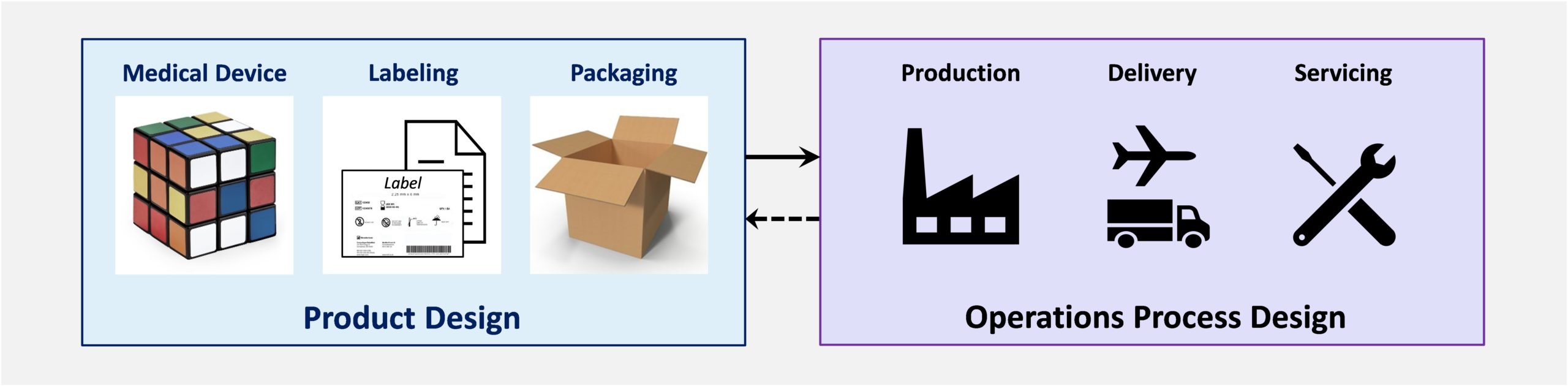
Design Controls includes both Product Design and Operations Process Design. Operations processes include:
- Medical Device Production – purchasing, manufacturing, remanufacturing, labeling, packaging, cleaning, disinfection, sterilization
- Medical Device Delivery – storage, transportation, installation, customer service/education, decommissioning & disposal
- Medical Device Servicing – maintenance and repair
Adjacent areas of expertise include:
- Usability Engineering – IEC 62366
- Software Life Cycle – IEC 62304
- OEM Product Development (with suppliers)
Products and Services
AganaMed offers the following products and services for medical device development:
- Educational Literature
- Educational Videos (e-learning)
- Training and Consulting (Product Development Services)
- Enabling Software – not yet saleable
These products and services are geared towards both experienced medical device industry professionals (who seek expert insights and best practices to improve or execute their existing product development processes) and new entrants to the medical device industry (who seek a practical introduction to medical device development). Intended customers include:
- Medical device Company Employees who engage in Product Development – project managers, design engineers, quality engineers, scientists, test engineers, manufacturing engineers, supplier engineers, service engineers, clinical representatives, regulatory representatives, marketing representatives
- Medical device Inventors – Healthcare providers (doctors, nurses, first responders), engineers, designers, hobbyists
- Medical device Educators
- Medical device Students
- Medical device Consultants
- Medical device Auditors
- Medical device Analysts
- Medical device Regulators
- Medical device Lawyers
- Medical device Investors
Contact
Email: support@aganamed.com
Mailing address:
AganaMed LLC 268 Bush St #4320 San Francisco, CA 94104 USA
Newsletter: Click here to sign up for the AganaMed eNewsletter (free).
