Glossary
Selected key terms pertaining to medical device development are listed below with References.
Key Terms
[FDA 21CFR801, FDA 21CFR803, FDA 21CFR806, FDA 21CFR810, FDA 21CFR814, FDA 21CFR820, FDA 21CFR821, FDA 21CFR822, FDA 21CFR830]
[EU MDR 2017/745, EU IVDR 2017/746]
[FDA website: Who Must Register...]
Note: All aspects of usability, including effectiveness, efficiency and user satisfaction, can either increase or decrease safety.
[IEC 62366-1:2015]
Note 1: Achieving adequate usability can result in acceptable risk related to use.
[IEC 62366-1:2015]
Note 2: Also known as human factors engineering (HFE)
[IEC 62366-1:2015]
[IEC 62366-1:2015]
Note: The conditions of use or attributes of the use environment can include hygienic requirements, frequency of use, location, lighting, noise, temperature, mobility, and degree of internationalization.
[IEC 62366-1:2015]
Note 1: Use error includes the inability of the user to complete a task.
Note 2: Use errors can result from a mismatch between the characteristics of the user, user interface, task, or use environment.
Note 3: USERS might be aware or unaware that a use error has occurred.
Note 4: An unexpected physiological response of the patient is not by itself considered use error.
Note 5: A malfunction of a medical device that causes an unexpected result is not considered a use error.
Note 6: The figure below shows the relationships of the types of use.
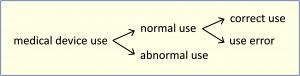
[IEC 62366-1:2015, ISO 14971:2019]
- a user who performs all or part of an examination (“analytical use”); and
- a clinician who receives, interprets and acts on the examination results (“clinical use”).
Note: In the case of IVD medical devices intended for self-testing, the patient can be the only user.
[ISO/TR 24971:2020]
Note 1: There can be more than one user of a medical device.
Note 2: Common users include clinicians, patients, cleaners, maintenance and service personnel.
[IEC 62366-1:2015]
Note 1: Accompanying documentation is considered part of the medical device and its user interface.
Note 2: User interface includes all the elements of the medical device with which the user interacts including the physical aspects of the medical device as well as visual, auditory, tactile displays and is not limited to a software interface.
Note 3: For the purposes of this standard, a system of medical devices can be treated as a single user interface.
[IEC 62366-1:2015]
Note: "Other customers" include other people who interact with the medical device during use and stakeholders who do not directly interact with the medical device such as post-production monitoring personnel, administrative personnel, regulatory bodies, and business stakeholders.
Medical device developers are strongly encouraged to consult the listed reference for each key term to fully understand the context behind the definition and its intended application.
Definitions from the FDA Code of Federal Regulation (CFR) are listed without dates and are accurate through December 2020. Consult the listed FDA reference (online) to confirm the latest definition.
