What is a Medical Device?
Tongue depressors, hospital beds, surgical scalpels, endoscopic cameras, infusion pumps, dental implants, and programmable pacemakers are examples of medical devices. The international standard ISO 13485 defines a medical device according to its intended use as an:
instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use, software, material or other similar or related article, intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the specific medical purpose(s) of:
– diagnosis, prevention, monitoring, treatment or alleviation of disease;
– diagnosis, monitoring, treatment, alleviation of or compensation for an injury;
– investigation, replacement, modification, or support of the anatomy or of a physiological process;
– supporting or sustaining life;
– control of conception,
– disinfection of medical devices;
– providing information by means of in vitro examination of specimens derived from the human body;
and does not achieve its primary intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its intended function by such means
Note: Products which may be considered to be medical devices in some jurisdictions but not in others include:
– disinfection substances;
– aids for persons with disabilities;
– devices incorporating animal and/or human tissues;
– devices for in vitro fertilization or assisted reproduction technologies.
ISO 13485:2016
Blood test kits, HIV test kits, and pregnancy self-test kits are examples of in vitro diagnostic (IVD) medical devices. ISO 20916 defines an in vitro diagnostic medical device as a:
medical device, whether used alone or in combination, intended by the manufacturer for the in vitro examination of specimens derived from the human body solely or principally to provide information for diagnostic, monitoring, or compatibility purposes
Note: IVD medical devices include reagents, calibrators, control materials, specimen receptacles, software and related instruments or apparatus or other articles and are used, for example, for the following test purposes: diagnosis, aid to diagnosis, screening, monitoring, predisposition, prognosis, prediction, determination of physiological status.
ISO 20916:2019
The global market for medical devices is over $500 billion (annual) and keeps growing, especially in emerging markets. The USA is the largest market (40% of global sales), followed by Europe (25%), and Japan (15%). Most medical device companies are small (less than 20 employees), but the top 1% of companies own over 83% of industry assets and an even larger share of global profits.
For an introduction to medical devices, view online video Course 110 – Medical Devices.
Medical Device Development
Every individual medical device undergoes a life-cycle consisting of some or all of the medical device life-cycle stages shown below:
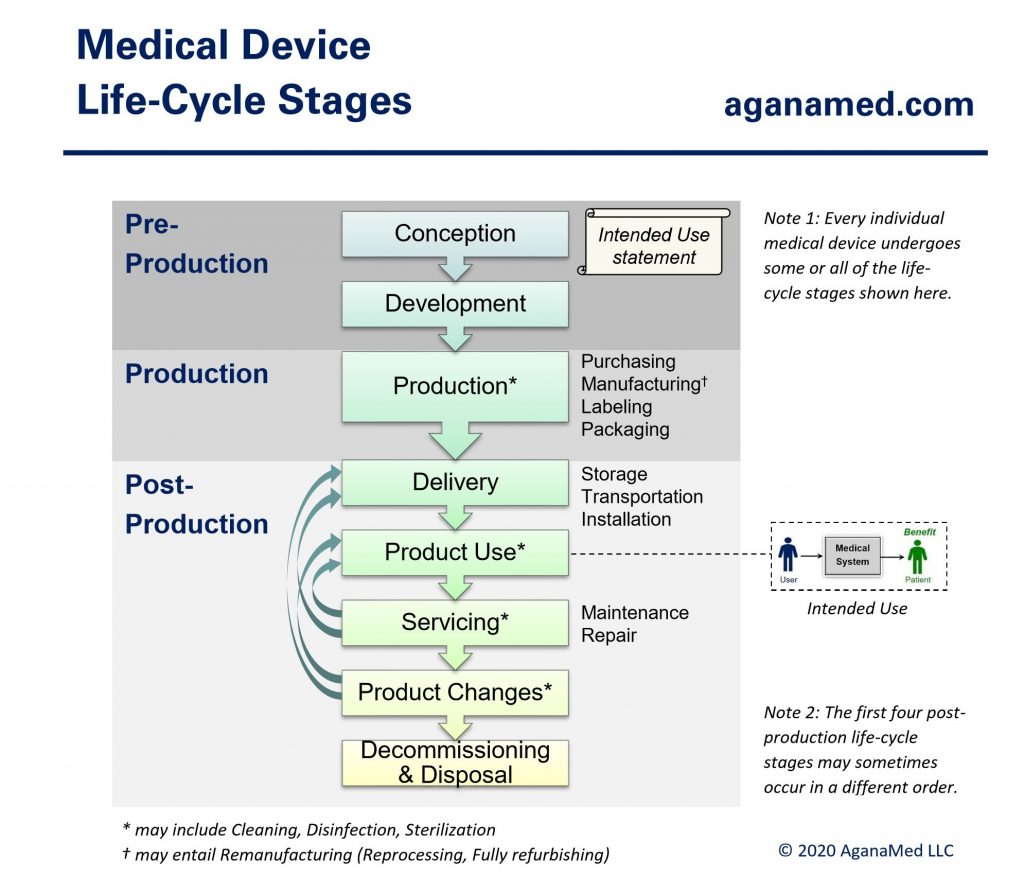
Companies (manufacturers) that develop medical devices often use product development life-cycle phases as a project management tool to indicate the maturity of the entire medical device product line. Hence, from the manufacturer’s point of view, medical device development consists of the product development life-cycle phases shown below:
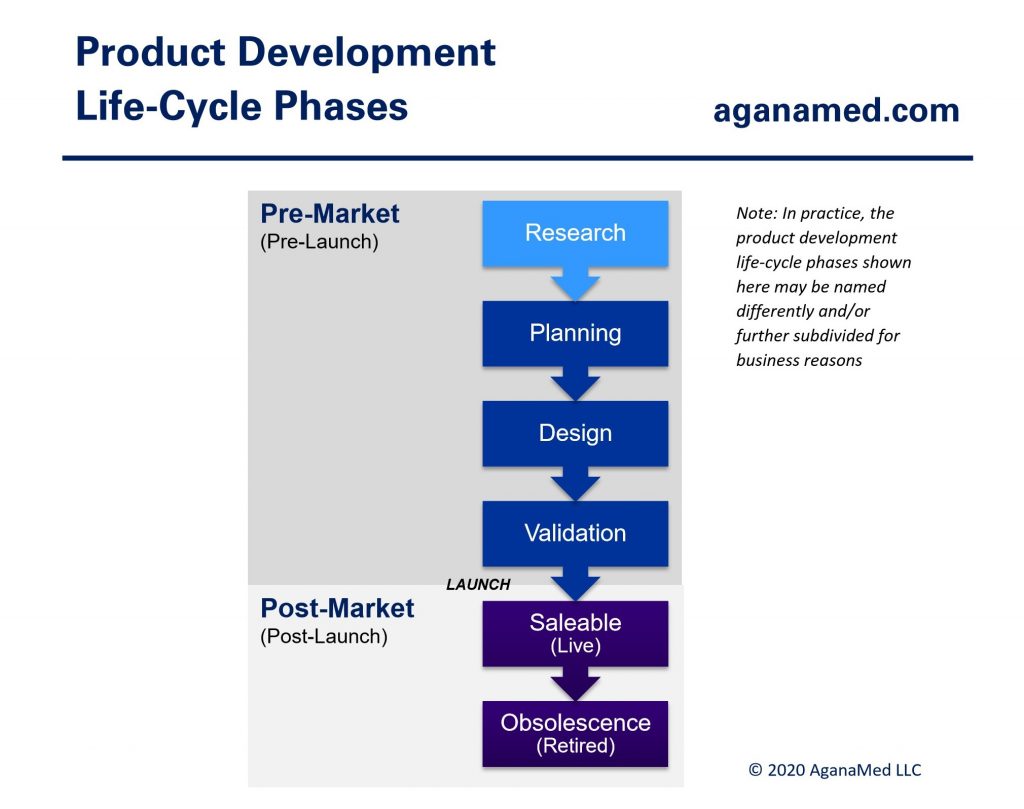
Different companies may combine, subdivide or name these life-cycle phases differently than what is shown above, but the life-cycle concepts are the same throughout the medical device industry.
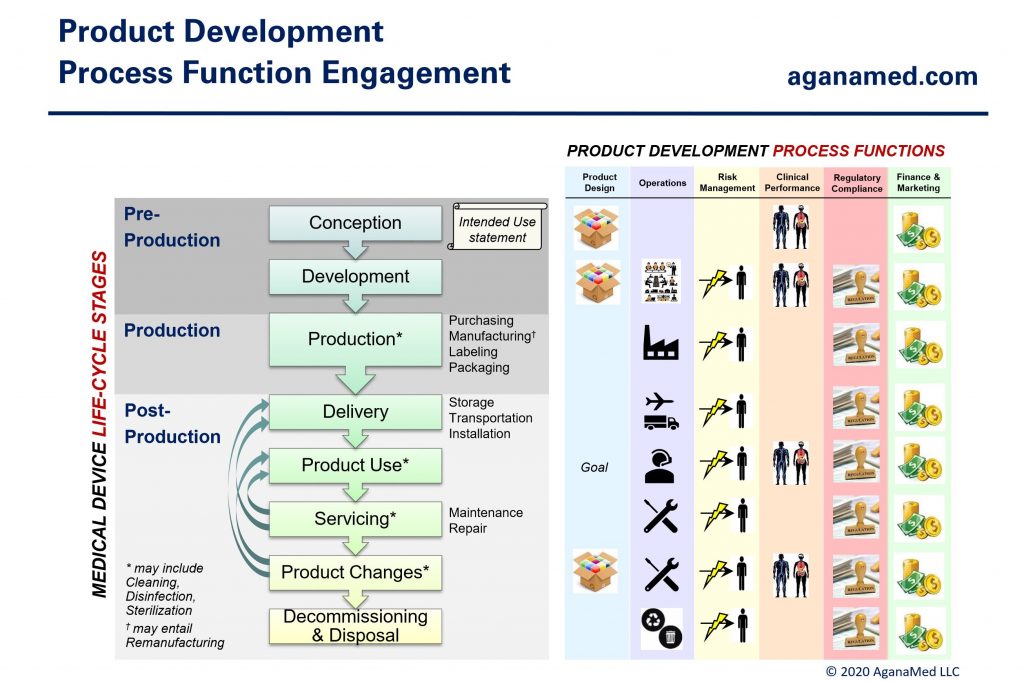
Medical device development activities can be divided into the 6 product development process functions shown below:
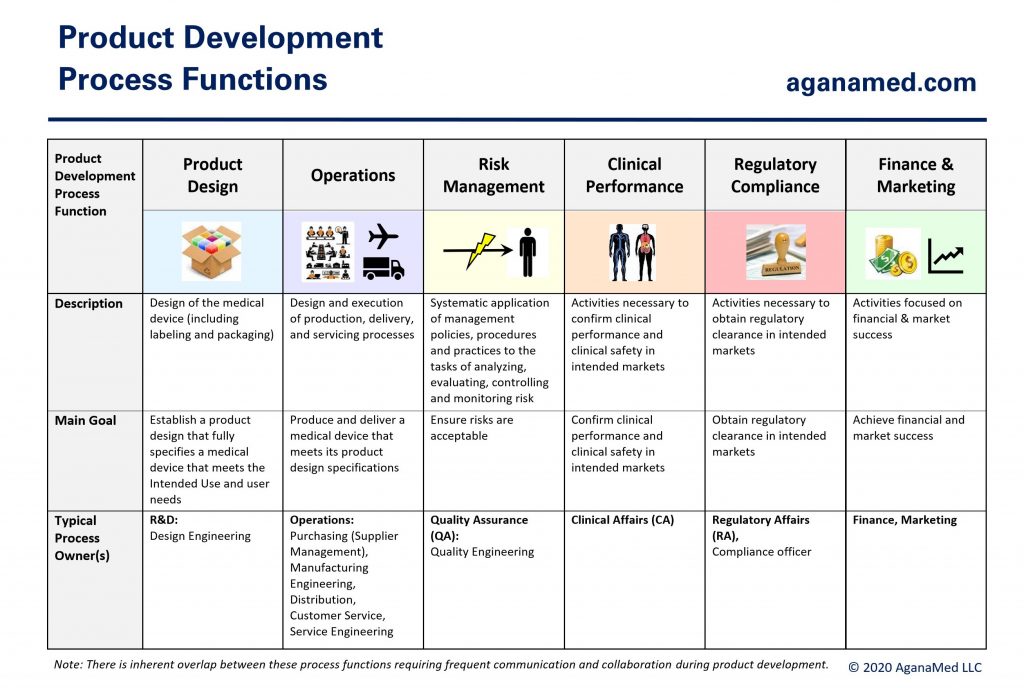
All 6 process functions must engage throughout the product development life-cycle, but the amount of work, and therefore, the level of engagement by each process function varies greatly depending on the nature of the medical device. Figure 4 below shows the basic structure of the medical device development process:
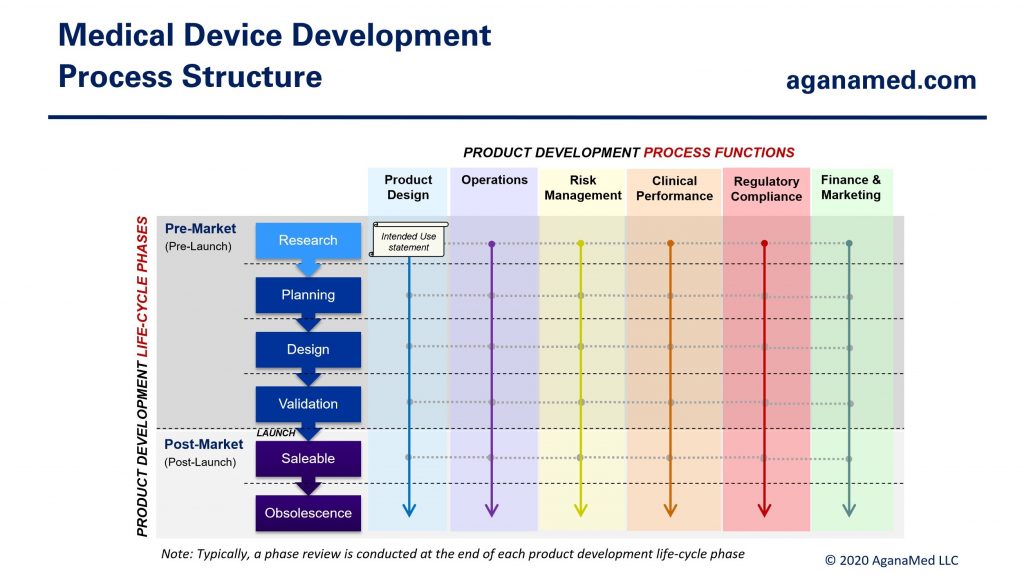
Figure 5 shows the typical engagement of each process function during the medical device life-cycle (from Figure 1):
For an introduction to medical device development, view online video Course 120 – Medical Device Development.
Medical Device Due Diligence
Companies and investors may need to conduct due diligence (objective research) on an existing medical device or medical device company during the following activities:
- Acquisition or assessment of a medical device company
- Acquisition, assessment, or licensing of a medical device or medical device technology
A practical understanding of medical device development is a prerequisite for effective due diligence.
Medical Device Terminology
Use of correct terminology is vital during medical device development. This task is made somewhat difficult because different regulatory jurisdictions often use (slightly) different terminology, necessitating medical device developers to deftly switch between different definitions for the same term. For benchmarking and comparison purposes, selected key terms pertaining to medical device development are listed in the Glossary with References.
File Download
Visit the File Download page to download PDF files of the figures displayed on this website.
Educational Literature Information form
For information or comments about educational literature or to request specific topics, submit the online form below:
