AganaMed online Educational Videos cover a wide range of Medical Device Development topics. Online videos can be viewed anytime—with pause and rewind—so viewers can consume the subject matter at their preferred pace and convenience for educational and/or training purposes (e-learning).
For more in-depth and interactive live trainings, visit the Training and Consulting page.
Training Curriculum
The AganaMed training curriculum for medical device development is shown below:
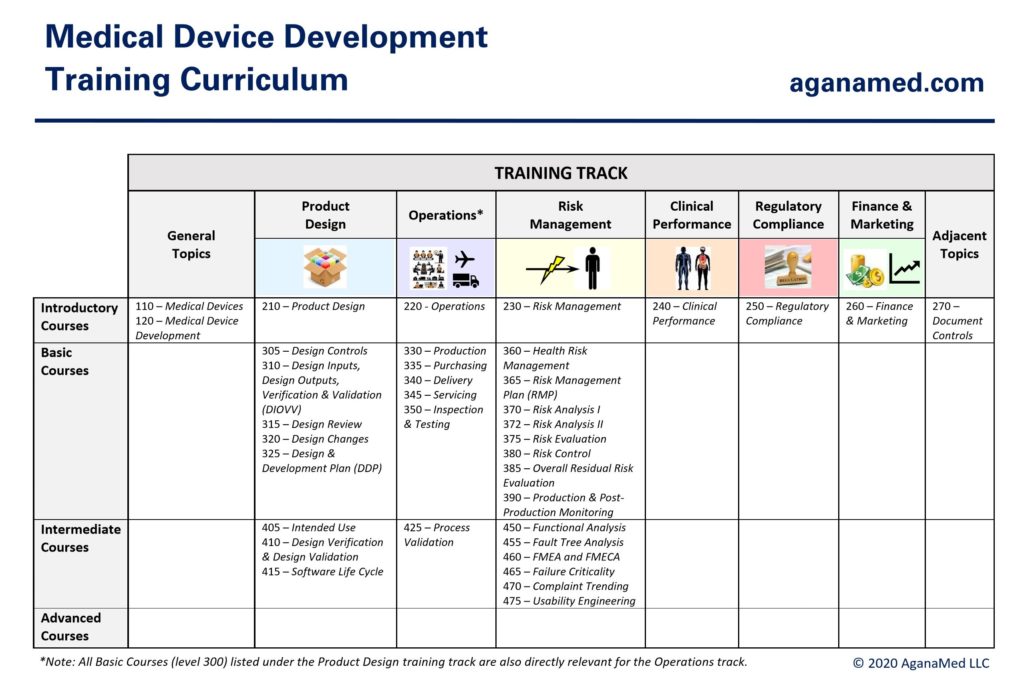
The training curriculum will be updated with new and revised videos on an ongoing basis to reflect the practical state of the art of medical device development. 5 hours of video is currently available (with membership).
Training Videos
by Course Level
- Introductory Courses (Level 100 and 200) – free preview available
- Basic Courses (Level 300)
- Intermediate Courses (Level 400) – will be available soon
by Training Track
- General Topics – free preview available
- Product Design – free preview available
- Operations – free preview available
- Risk Management – free preview available
- Clinical Performance
- Regulatory Compliance
- Finance and Marketing
- Adjacent Topics
Training Assignments
The Introductory Courses are non-technical and intended for a general audience (everyone) whereas the Basic Courses are somewhat technical and intended for medical device developers (or those who plan to engage in medical development soon).
Typical training course assignments for each role on the medical device development Project Team are shown below:
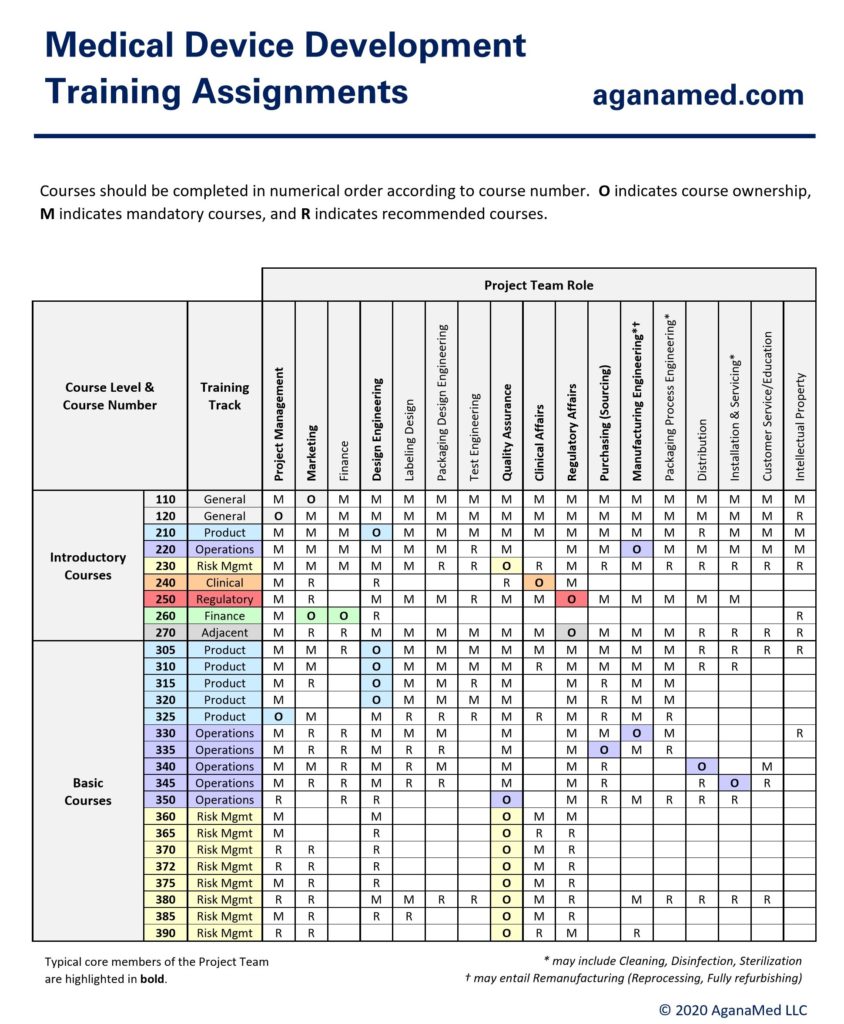
In medium-to-large companies in which top management is not part of the project team, only the first 1-2 courses are recommended for top management under normal (non-adverse) conditions.
Visit the File Download page to download PDF files of the figures displayed above.
Educational Videos Information Form
For information about educational videos or to request specific topics, submit the online form below:
