Glossary
Selected key terms pertaining to medical device development are listed below with References.
Key Terms
There are currently 4 key terms in this directory beginning with the letter N.
normal use
operation, including routine inspection and adjustments by any user, and stand-by, according to the instructions for use or in accordance with generally accepted practice for those medical devices provided without instructions for use
Note 1: Normal use should not be confused with intended use. While both include the concept of use as intended by the manufacturer, intended use focuses on the medical purpose while normal use incorporates not only the medical purpose, but maintenance, transport, etc. as well.
Note 2: Use error can occur in normal use.
Note 3: Medical devices that can be used safely without instructions for use are exempted from having instructions for use by some authorities with jurisdiction.
Note 4: The figure below shows the relationships of the types of use.
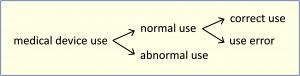
Note 1: Normal use should not be confused with intended use. While both include the concept of use as intended by the manufacturer, intended use focuses on the medical purpose while normal use incorporates not only the medical purpose, but maintenance, transport, etc. as well.
Note 2: Use error can occur in normal use.
Note 3: Medical devices that can be used safely without instructions for use are exempted from having instructions for use by some authorities with jurisdiction.
Note 4: The figure below shows the relationships of the types of use.

[IEC 62366-1:2015]
Medical device developers are strongly encouraged to consult the listed reference for each key term to fully understand the context behind the definition and its intended application.
Definitions from the FDA Code of Federal Regulation (CFR) are listed without dates and are accurate through December 2020. Consult the listed FDA reference (online) to confirm the latest definition.
